Tarling Lab Research
Overview
Lipids are not only essential building blocks for cells, but they are also important structural and signaling molecules. Dysregulation of cellular and/or plasma lipid levels results in different diseases that can affect multiple different organ systems such as diabetes, cardiovascular disease, fibrosis, and pulmonary alveolar proteinosis. We are interested in mechanisms that control cellular and whole-body lipid homeostasis, particularly in cardiopulmonary diseases.
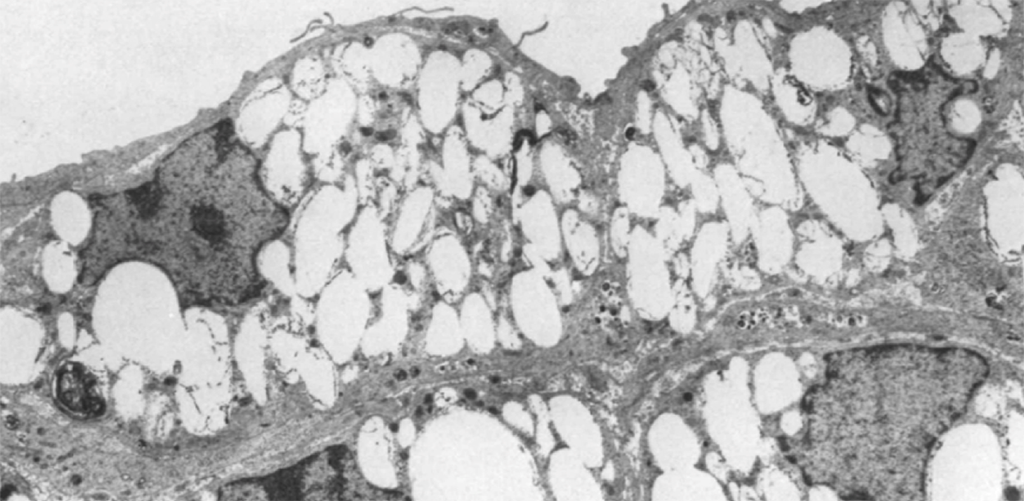
Atherosclerotic Lesion with Macrophage Foam Cells
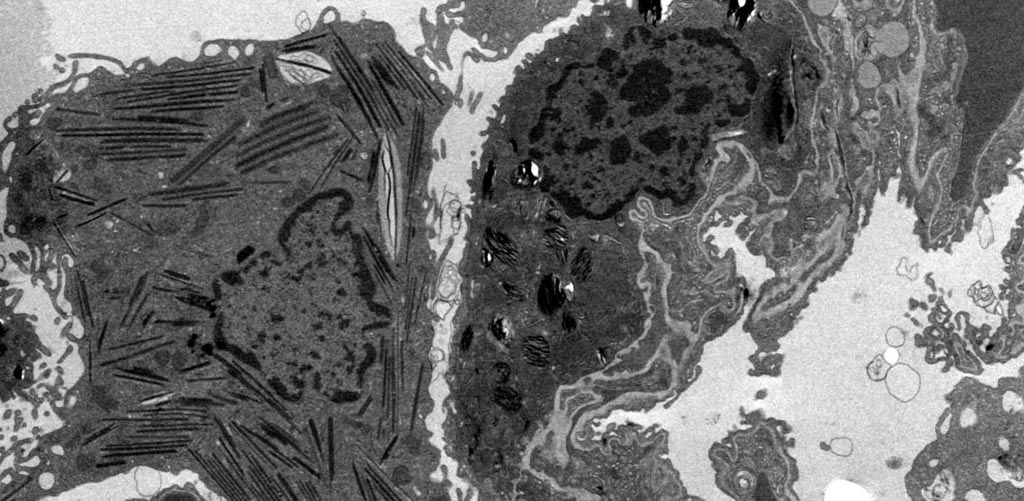
Lipid Accumulation in Lung Macrophages
From Bedside to Bench to Bedside: Towards Precision Medicine for Lung Disease
Precision medicine considers the variability among individuals and allows for the more accurate prediction of which treatment/prevention strategies will work in a given patient. Pulmonary alveolar proteinosis (PAP) is a rare lung syndrome caused by the accumulation of surfactant and lipids in the alveolar space. PAP typically occurs in the prime of life (age 30-50), affecting people of all ethnicities and geographic locations, regardless of socioeconomic status. There is no cure and there are no FDA approved therapies for PAP. The standard of care is whole lung lavage, an invasive high-risk procedure. Our research shows that cholesterol is the predominant material accumulating in PAP alveolar macrophages and that medications such as Pioglitazone (currently in clinical trials for PAP) and Statins, which reduce alveolar macrophage cholesterol content and disease severity may be novel therapies for PAP. PAP patients have limited options for therapy, and are often refractory to treatments. We are using state of the art lipidomic analyses to understand the pathologic lipid species in PAP alveolar macrophages and how patient-derived cells respond to specific therapies to help identify which treatment(s) will be most effective in individual PAP patients.
Crosstalk Between Lipid Metabolism, Inflammation, and Immunity
The ATP-Binding Cassette Transport G1 (ABCG1) is a lipid transporter expressed in many cells and tissues, including macrophages, B cells, and lung epithelial cells. We have focused on investigating the role of the ATP Binding Cassette Transporter ABCG1 in lipid metabolism, inflammation, atherosclerosis and innate immunity. We are currently studying the role of ABCG1 in pulmonary lipid homeostasis and immunity, and in human disease. We have established animal models of loss of ABCG1 (whole-body and tissue specific), and have been able to demonstrate that alterations in intracellular cholesterol and phospholipids affects multiple pathways, including inflammatory signaling and immune cell function. We have also identified single nucleotide polymorphisms in the ABCG1 locus in patients with the rare lung syndrome Pulmonary Alveolar Proteinosis (PAP). Understanding the interplay between lipids and immune responses will help us in identifying possible new targets for therapeutic intervention.
Identifying Novel Regulators of Lipid Metabolism
We are interested in understanding how lipids are handled, transported, and stored in normal and diseased states. We study known regulators of lipid homoestasis to delineate molecular mechanisms. We use a combination of targeted and unbiased screening approaches to identify new genes that regulate lipid metabolism and cholesterol homeostasis. We have explored the natural genetic variation in 103 strains of mice and are using multi-omic analyses to identify genes and loci that control lipid metabolism. We have recently identified a novel gene that regulates plasma LDL cholesterol and glucose levels and is also a locus for LDL cholesterol levels and body mass index in human Genome-wide Association Studies. Our focus is on determining the molecular mechanism and structural requirements of the changes in plasma cholesterol levels, and the therapeutic potential of ASO silencing agents in disease models of atherosclerosis and diabetes.